Metals and alloys
The development of improved metallic materials is a vital activity at the leading edge of science and technology. Metals offer various combinations of properties and reliability at a cost which is affordable. They are versatile because subtle changes in their microstructure can cause dramatic variations in their properties. An understanding of the development of microstructure in metals, rooted in thermodynamics, crystallography and kinetic phenomena is essential for the materials scientist.
Alloys can blend the properties of two or more metals to create a hybrid metal that is more cost effective, stronger, more durable, and overall better suited to its intended purpose than the pure metals used to create the compound. With emerging requirement of designing new materials capable of sustaining high strain rate and severe operating conditions with reduced wastage of cost, energy, and material, it has become an important issue to develop full understanding of the nature of enhanced mechanical properties of the materials. New materials that can be tailored for individual applications are always in a constant demand. As the range of uses for powder metallurgy, hard metals and electronic materials expands, customer requirements are causing materials companies to come up with new products that have the required properties.
Metals and semiconductors play an important role in the present world as evidenced by their variety of applications. Hence, a study on some important metals, alloys and semiconducting systems is essential in terms of the local structure and the average structure which are completely different. The usual methods of analysis using structural refinement of X-ray or neutron data will give only the average structure of the materials under investigation. The studies on the local structure of materials seem to be rare because of the complexity of the problem. There is only limited information available on the investigations of materials in terms of the local structure. Numerous research papers are being published every year based on powder as well as single crystal X-ray diffraction data. The structures reported using those data are only average structures. Since, the analysis of local structure needs highly precise data up to maximum possible Bragg angle, accurate refinement of the data is limited. Due to the complexity of the problem, tasks of acquirement of precise X-ray data from the samples, and the computational incapabilities, local and average structural analysis has not been much explored. Atomic ordering is closely related to the materials’ electronic and magnetic properties. Although the physical properties of alloys are closely related to their electronic structures, studies on the charge transfer and hybridization of the electronic states are still insufficient.
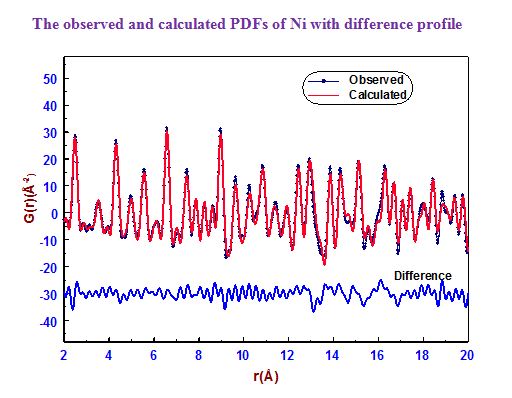
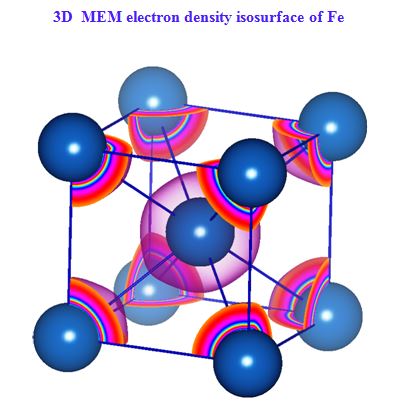
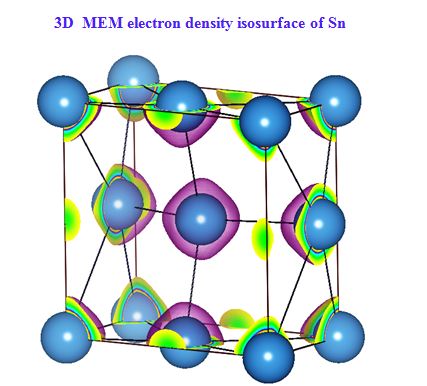
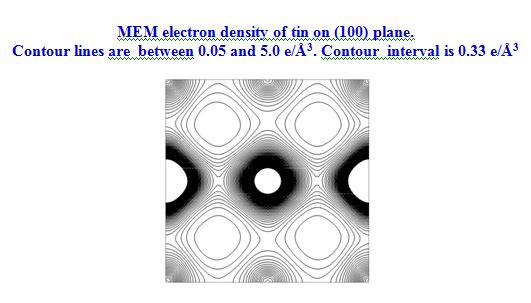
In our investigation, apart from pure metals, the local and average structures of doped metals and alloys are carried out with various doping concentrations. The average structure has been studied using both single crystal and powder X-ray diffraction data in some cases. The bonding and electron density distribution of the host as well as dopant atoms has been studied using tools like MEM (maximum Entropy Method) and multipole analysis. For powder analysis, Rietveld refinement technique (for average structure) and Pair Distribution Function (for local structure) have been used. Effects on the electron density distribution of ball milling has been analysed in this work.
The metals and alloys analysed are Sodium (Na),Vanadium (V),Magnesium (Mg), Aluminium (Al),Titanium (Ti), Iron (Fe), Nickel (Ni), Copper (Cu), Zinc (Zn), Tin (Sn), Tellurium (Te), cobalt aluminium (CoAl), nickel aluminium (NiAl), Iron nickel (FeNi), nickel chromium (Ni80Cr20) , Sodium chloride with iron impurities (Na1-xAgxCl), Aluminium, with iron impurities (0.215 wt % Fe and 0.304 wt % Fe), doped and undoped semiconductor gallium arsenide (n – GaAs).
Research Materials
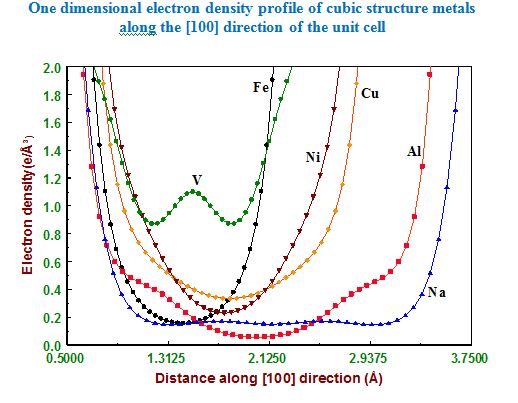
Na
V
Mg
Al
Ti
Fe
Ni
Cu
Zn
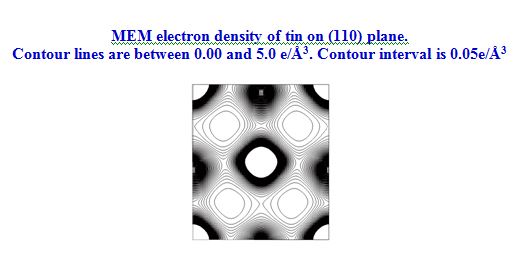
Sn
Te
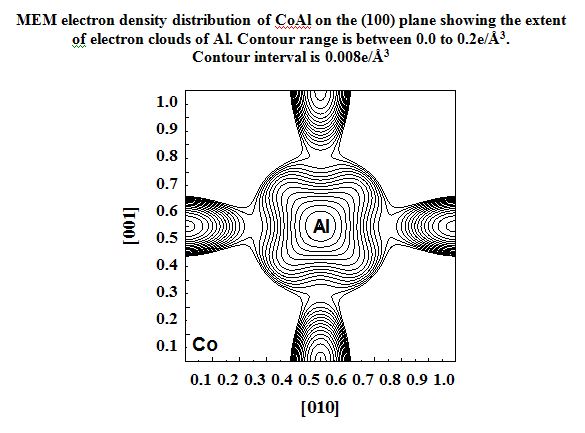
CoAl
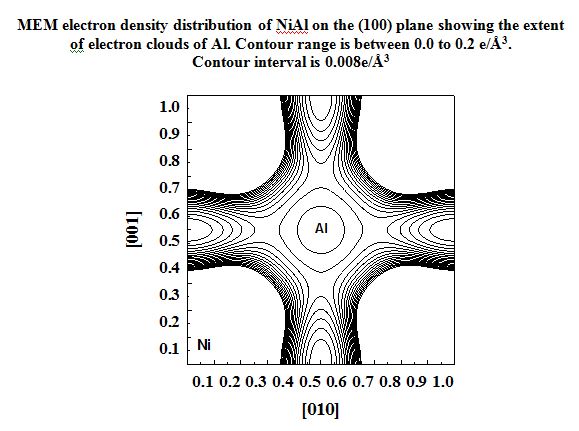
NiAl
FeNi
Ni80Cr20)
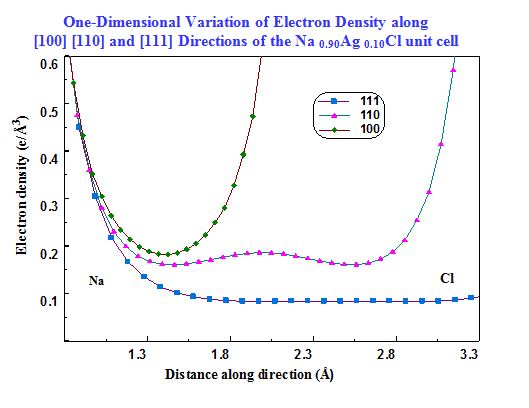
Na1-xAgxCl
Aluminium with iron impurities (0.215 wt % Fe and 0.304 wt % Fe),
n-GaAs